We conduct high-quality clinical research to generate robust data, improve patient quality of life, and strengthen health systems across the region.
We conduct high-quality clinical research to generate robust data, improve patient quality of life, and strengthen health systems across the region.
About Us
Accelerating Innovative Treatments
and Driving Impact in Latin America
Our goal is twofold: to accelerate the development of innovative treatments and to create a lasting impact on health systems and communities across Latin America.
EndPoints is a network of research sites in Latin America, specializing in clinical, epidemiological, and public health studies. We deliver innovative solutions and robust processes to ensure high-quality data and outcomes.
We partner with CROs, biotechs, pharma, and public health organizations to execute reliable, high-impact clinical trials.
With +100 trials conducted and over 50,000 participants enrolled, we have collaborated with +30 global sponsors and CROs.
Our network is driven by +400 skilled professionals with strong regional and scientific expertise.

Our Motivation
Transforming science into real solutions for health
We drive clinical innovation to generate reliable evidence, improve people’s quality of life, and strengthen health systems across Latin America.
About Us
Accelerating Innovative Treatments
and Driving Impact in Latin America
Our goal is twofold: to accelerate the development of innovative treatments and to create a lasting impact on health systems and communities across Latin America.
EndPoints is a network of research sites in Latin America, specializing in clinical, epidemiological, and public health studies. We deliver innovative solutions and robust processes to ensure high-quality data and outcomes.
We partner with CROs, biotechs, pharma, and public health organizations to execute reliable, high-impact clinical trials.
With +100 trials conducted and over 50,000 participants enrolled, we have collaborated with +30 global sponsors and CROs.
Our network is driven by +400 skilled professionals with strong regional and scientific expertise.

Our Motivation
Transforming science into real solutions for health
We drive clinical innovation to generate reliable evidence, improve people’s quality of life, and strengthen health systems across Latin America.
Our Strengths
The Strength of a Network
Powered by the Local Intelligence of Each Site

Faster and more accurate feasibility through streamlined site assessment

Regulatory intelligence that accelerates outcomes by simplifying approvals and reducing timelines.

A single point of contact ensures smooth and efficient communication

Sites working as a network - rather than individually - foster a stronger and more standardized study environment
Our Strengths
The Strength of a Network
Powered by the Local Intelligence of Each Site

Faster and more accurate feasibility through streamlined site assessment

Regulatory intelligence that accelerates outcomes by simplifying approvals and reducing timelines.

A single point of contact ensures smooth and efficient communication

Sites working as a network - rather than individually - foster a stronger and more standardized study environment
Our Experience
+0
Clinical Trials
+0
Subjects
+0
Sponsors and CROs
+0
Professionals
0
Clinical Research Centers
+0 years
Experience and Achievements
<0%
Drop-Off Rate
Study Phase
0%
Phase III
0%
Phase II
0%
Phase II/III
0%
Epidemiological
0%
Observational
0%
Phase I/II & IV
0%
Phase I & Medical Device
0%
Phase 0
Our Experience
+0
Clinical Trials
+0
Subjects
+0
Sponsors and CROs
+0
Professionals
0
Clinical Research Centers
+0 years
Experience and Achievements
<0%
Drop-Off Rate
Study Phase
0%
Phase III
0%
Phase II
0%
Phase II/III
0%
Epidemiological
0%
Observational
0%
Phase I/II & IV
0%
Phase I & Medical Device
0%
Phase 0
































































































































Our Impact
Some of our most notable achievements include

Participation in the development of over 15 vaccines currently approved

Collaboration with organizations such as the World Health Organization (WHO) and NIH

Trials completed with no critical findings in international audits, including those by the FDA
Studies conducted in partnership with companies such as Pfizer, GSK, Janssen, Sanofi, and Novavax

Record-breaking enrollment in multicenter studies across Latin America for COVID-19, norovirus, and RSV
Our Impact
Some of our most notable achievements include

Participation in the development of over 15 vaccines currently approved

Collaboration with organizations such as the World Health Organization (WHO) and NIH

Trials completed with no critical findings in international audits, including those by the FDA

Studies conducted in partnership with companies such as Pfizer, GSK, Janssen, Sanofi, and Novavax

Record-breaking enrollment in multicenter studies across Latin America for COVID-19, norovirus, and RSV
Our Therapeutic Areas
Infectious Diseases and Vaccines
COVID-19, RSV, Dengue, Poliovirus, Norovirus, Influenza, Pneumococcus, Chikungunya, HIV, Meningitis, Hepatitis A, Herpesvirus, HPV, Pertussis, Varicella, Zika Virus, others.
Cardiology
Hypertension, Dyslipidemia, Heart failure, Arrhythmias, Post-acute MI, Devices
Endocrinology and Metabolism
Diabetes, Obesity, Other Hormonal Disorders
Neurology and Psychiatry
Depression, Epilepsy, Migraine, Alzheimer’s Disease, Parkinson’s Disease
Oncology
Observational cancer studies (breast, cervical), Solid Tumor, Radiation Oncology
Medical Devices
Remote monitoring devices (BP, ECG, glucose), Diagnostic Devices, Neurological Devices, Women’s health diagnostics, Wearables
Our Therapeutic Areas
Infectious Diseases and Vaccines
COVID-19, RSV, Dengue, Poliovirus, Norovirus, Influenza, Pneumococcus, Chikungunya, HIV, Meningitis, Hepatitis A, Herpesvirus, HPV, Pertussis, Varicella, Zika Virus, others.
Cardiology
Hypertension, Dyslipidemia, Heart failure, Arrhythmias, Post-acute MI, Devices
Endocrinology and Metabolism
Diabetes, Obesity, Other Hormonal Disorders
Neurology and Psychiatry
Depression, Epilepsy, Migraine, Alzheimer’s Disease, Parkinson’s Disease
Oncology
Observational cancer studies (breast, cervical), Solid Tumor, Radiation Oncology
Medical Devices
Remote monitoring devices (BP, ECG, glucose), Diagnostic Devices, Neurological Devices, Women’s health diagnostics, Wearables
Our Principal Investigators

Dr. Rodrigo DeAntonio
▾- MD, epidemiologist, Master in Health Economics, Doctor in Public Health.
- Executive and Scientific Director of the Cevaxin Research Center in Panama.
- Leads clinical and epidemiological studies for vaccine-preventable diseases.
- Research focus includes rotavirus, pneumococcus, meningococcus, HPV, pertussis, hepatitis, RSV, influenza, chickenpox, norovirus, dengue, and COVID-19.

Dr. Xavier Saez Llorens
▾- Medical degree from the University of Panama.
- Postdoctoral specialization in Pediatrics (Panama) and Infectious Diseases (University of Texas Southwestern Medical Center, USA).
- Professor of Pediatrics, Chief of Infectious Diseases, and Director of Clinical Research at Hospital del Niño Dr. José Renán Esquivel.
- Distinguished Investigator (Senacyt, Cevaxin), member of Panama’s National Ethics Committee.
- Honorary member of the Catalonian Institute of Biological Sciences.
- COVID vaccine advisor for the Panamanian government.

Dr. Eduardo Lopez-Medina
▾- Physician trained at Universidad del Valle (Cali, Colombia).
- Residency in Pediatrics at Miami Children’s Hospital.
- Fellowship in Pediatric Infectious Diseases at the University of Texas.
- Master’s in Epidemiology from the University of London.
- Completed a clinical research program at Harvard.
- Research focuses on vaccine-preventable diseases and infections in immunocompromised children.

Dr. Mario Melgar
▾- Pediatric Infectious Diseases specialist trained at Federico Gómez Children’s Hospital (Mexico).
- Head of the Pediatric Infectious Diseases Unit at Roosevelt Hospital and the National Pediatric Oncology Unit in Guatemala.
- Leads residency and master’s programs in Infectious Diseases at Roosevelt Hospital.
- Director of CECLISA, a research center conducting Phase I/II/III clinical trials in various areas including vaccines, infectious diseases, rheumatology, and oncology.

Dr. Pío López
▾- Infectious disease specialist and pediatrician. - General Director of the Center for Studies in Pediatric Infectious Diseases (CEIP), Cali, Colombia.
- Former President of the Colombian Association of Infectious Diseases (ACIN) for the terms 2015–2017 and 2017–2019.
- Former President of the Latin American Society for Pediatric Infectious Diseases (SLIPE) from 2019 to 2021.

Dr. Camilo Rodriguez
▾- Internal medicine and pulmonology specialist, with a master's degree in pulmonary hypertension from the University of Bologna. Former pulmonary transplant preceptor at the Cleveland Clinic.
- Currently works as a pulmonologist at Fundación Cardioinfantil and serves as Head of the Outpatient Service at Fundación Neumológica Colombiana.
Our Principal Investigators

Dr. Rodrigo DeAntonio
▾- MD, epidemiologist, Master in Health Economics, Doctor in Public Health.
- Executive and Scientific Director of the Cevaxin Research Center in Panama.
- Leads clinical and epidemiological studies for vaccine-preventable diseases.
- Research focus includes rotavirus, pneumococcus, meningococcus, HPV, pertussis, hepatitis, RSV, influenza, chickenpox, norovirus, dengue, and COVID-19.

Dr. Xavier Saez Llorens
▾- Medical degree from the University of Panama.
- Postdoctoral specialization in Pediatrics (Panama) and Infectious Diseases (University of Texas Southwestern Medical Center, USA).
- Professor of Pediatrics, Chief of Infectious Diseases, and Director of Clinical Research at Hospital del Niño Dr. José Renán Esquivel.
- Distinguished Investigator (Senacyt, Cevaxin), member of Panama’s National Ethics Committee.
- Honorary member of the Catalonian Institute of Biological Sciences.
- COVID vaccine advisor for the Panamanian government.

Dr. Eduardo Lopez-Medina
▾- Physician trained at Universidad del Valle (Cali, Colombia).
- Residency in Pediatrics at Miami Children’s Hospital.
- Fellowship in Pediatric Infectious Diseases at the University of Texas.
- Master’s in Epidemiology from the University of London.
- Completed a clinical research program at Harvard.
- Research focuses on vaccine-preventable diseases and infections in immunocompromised children.

Dr. Mario Melgar
▾- Pediatric Infectious Diseases specialist trained at Federico Gómez Children’s Hospital (Mexico).
- Head of the Pediatric Infectious Diseases Unit at Roosevelt Hospital and the National Pediatric Oncology Unit in Guatemala.
- Leads residency and master’s programs in Infectious Diseases at Roosevelt Hospital.
- Director of CECLISA, a research center conducting Phase I/II/III clinical trials in various areas including vaccines, infectious diseases, rheumatology, and oncology.

Dr. Pío López
▾- Infectious disease specialist and pediatrician. - General Director of the Center for Studies in Pediatric Infectious Diseases (CEIP), Cali, Colombia.
- Former President of the Colombian Association of Infectious Diseases (ACIN) for the terms 2015–2017 and 2017–2019.
- Former President of the Latin American Society for Pediatric Infectious Diseases (SLIPE) from 2019 to 2021.

Dr. Camilo Rodriguez
▾- Internal medicine and pulmonology specialist, with a master's degree in pulmonary hypertension from the University of Bologna. Former pulmonary transplant preceptor at the Cleveland Clinic.
- Currently works as a pulmonologist at Fundación Cardioinfantil and serves as Head of the Outpatient Service at Fundación Neumológica Colombiana.
Our Executive Team

Cristina Gómez
Human Resources
Human Resources executive with over 15 years of experience leading talent, culture, and organizational transformation strategies in global companies across the healthcare, clinical research, and technology sectors. She has held regional and global roles at multinational organizations such as Sanofi, VaxTrials, and Emmes, driving HR operating models, talent attraction and development, and culture change initiatives. Cristina holds a Bachelor's in Psychology and a Master's in Strategic Thinking, and is recognized for her innovative mindset, approachable leadership, and strong business acumen.

Maria Cecilia Laxalde
Director of Operations
Maria Cecilia Laxalde is a Clinical Research Operations professional with over 24 years of experience in the pharmaceutical industry and leading Contract Research Organizations (CROs). She also holds a technical degree in Human Resources Management, which supports her strong focus on team leadership, organizational development, and strategic planning. Her career includes regional and global leadership positions overseeing Phase I–IV clinical trials across Latin America, North America, Europe, and Asia-Pacific. She has held key roles in Clinical Operations, Quality Assurance, CRA Development, and Global Trial Management, working in collaboration with multidisciplinary teams and cross-functional stakeholders. Currently serving as Director of Operations, Maria Cecilia designs and executes comprehensive operational strategies to ensure regulatory compliance, operational efficiency, and high-quality performance. Her role involves aligning clinical operations with corporate strategy, fostering strategic relationships that generate high impact, and driving the growth and expansion of the business. She is deeply committed to strengthening site capabilities for high-impact clinical research, ensuring operational excellence in clinical studies, continuously evaluating and improving performance, and expanding and fortifying the clinical research site network. Additionally, she promotes a culture of ethical conduct, continuous improvement, and operational excellence while fostering strong partnerships with sponsors and CROs.

Amanda Vieira de Aguiar
Director of Regulatory and Start-up
Amanda Vieira de Aguiar holds a degree in Pharmacy and Biochemistry from the University of São Paulo (USP) and brings over 27 years of experience in Clinical Research, with a strong background in both major CROs and the pharmaceutical industry. Throughout her career, she has established herself as a leader in clinical operations, with a particular focus on regulatory processes and start-up strategies for multicenter studies across Latin America. As Director of Regulatory and Start-up, Amanda is responsible for overseeing ethical and regulatory submissions, managing timelines, and implementing efficient processes aligned with both local and international requirements. Her work is defined by technical excellence, strategic vision, and a strong commitment to quality and regulatory compliance. Amanda has extensive experience in team and project management and plays an active role in talent development, process standardization, and continuous improvement initiatives. She is fluent in English and Spanish and has been a registered pharmacist with the Brazilian Federal Pharmacy Council since 2009.

Pablo Díaz del Castillo
Chief Finance Officer
Pablo Díaz del Castillo is an economist with an advance degree in corporate finance and over two decades of experience leading strategic and financial functions in high-impact organizations across Latin America. Throughout his career, he has played a key role in regional expansion, financial model structuring, operational transformation, and the execution of investment projects in sectors such as healthcare, clinical research, industry, consumer goods, and financial services. With a practical, results-oriented approach and a comprehensive business perspective, Pablo contributes to the Endpoints leadership team by aligning financial management with the company’s strategic goals for regional growth and consolidation.

Angel Huidobro
Director of Business Development EU & Asia
Angel is a global business leader with a career spanning corporate development, sales strategy, and international market expansion. With a PhD in Analytical Chemistry in Pharmaceutical Sciences, he began his career at Crucell, DSM, and Janssen Pharmaceuticals, gaining hands-on experience in pharmaceutical development and project management, which fueled his interest in commercial strategy. At VISUfarma, he served as CMC Manager in the European Supply Chain department, refining his expertise in operations and supply chain management. He later completed the Global Executive OneMBA at RSM Erasmus University (2019), enabling his transition into strategic leadership roles that bridge science and business. With a proven track record in the CRO and CDMO sectors, Angel now serves as Director of Business Development at endPoints, leading growth through strategic partnerships and global market expansion.
Our Executive Team

Cristina Gómez
Human Resources
Human Resources executive with over 15 years of experience leading talent, culture, and organizational transformation strategies in global companies across the healthcare, clinical research, and technology sectors. She has held regional and global roles at multinational organizations such as Sanofi, VaxTrials, and Emmes, driving HR operating models, talent attraction and development, and culture change initiatives. Cristina holds a Bachelor's in Psychology and a Master's in Strategic Thinking, and is recognized for her innovative mindset, approachable leadership, and strong business acumen.

Maria Cecilia Laxalde
Director of Operations
Maria Cecilia Laxalde is a Clinical Research Operations professional with over 24 years of experience in the pharmaceutical industry and leading Contract Research Organizations (CROs). She also holds a technical degree in Human Resources Management, which supports her strong focus on team leadership, organizational development, and strategic planning. Her career includes regional and global leadership positions overseeing Phase I–IV clinical trials across Latin America, North America, Europe, and Asia-Pacific. She has held key roles in Clinical Operations, Quality Assurance, CRA Development, and Global Trial Management, working in collaboration with multidisciplinary teams and cross-functional stakeholders. Currently serving as Director of Operations, Maria Cecilia designs and executes comprehensive operational strategies to ensure regulatory compliance, operational efficiency, and high-quality performance. Her role involves aligning clinical operations with corporate strategy, fostering strategic relationships that generate high impact, and driving the growth and expansion of the business. She is deeply committed to strengthening site capabilities for high-impact clinical research, ensuring operational excellence in clinical studies, continuously evaluating and improving performance, and expanding and fortifying the clinical research site network. Additionally, she promotes a culture of ethical conduct, continuous improvement, and operational excellence while fostering strong partnerships with sponsors and CROs.

Amanda Vieira de Aguiar
Director of Regulatory and Start-up
Amanda Vieira de Aguiar holds a degree in Pharmacy and Biochemistry from the University of São Paulo (USP) and brings over 27 years of experience in Clinical Research, with a strong background in both major CROs and the pharmaceutical industry. Throughout her career, she has established herself as a leader in clinical operations, with a particular focus on regulatory processes and start-up strategies for multicenter studies across Latin America. As Director of Regulatory and Start-up, Amanda is responsible for overseeing ethical and regulatory submissions, managing timelines, and implementing efficient processes aligned with both local and international requirements. Her work is defined by technical excellence, strategic vision, and a strong commitment to quality and regulatory compliance. Amanda has extensive experience in team and project management and plays an active role in talent development, process standardization, and continuous improvement initiatives. She is fluent in English and Spanish and has been a registered pharmacist with the Brazilian Federal Pharmacy Council since 2009.

Pablo Díaz del Castillo
Chief Finance Officer
Pablo Díaz del Castillo is an economist with an advance degree in corporate finance and over two decades of experience leading strategic and financial functions in high-impact organizations across Latin America. Throughout his career, he has played a key role in regional expansion, financial model structuring, operational transformation, and the execution of investment projects in sectors such as healthcare, clinical research, industry, consumer goods, and financial services. With a practical, results-oriented approach and a comprehensive business perspective, Pablo contributes to the Endpoints leadership team by aligning financial management with the company’s strategic goals for regional growth and consolidation.

Angel Huidobro
Director of Business Development EU & Asia
Angel is a global business leader with a career spanning corporate development, sales strategy, and international market expansion. With a PhD in Analytical Chemistry in Pharmaceutical Sciences, he began his career at Crucell, DSM, and Janssen Pharmaceuticals, gaining hands-on experience in pharmaceutical development and project management, which fueled his interest in commercial strategy. At VISUfarma, he served as CMC Manager in the European Supply Chain department, refining his expertise in operations and supply chain management. He later completed the Global Executive OneMBA at RSM Erasmus University (2019), enabling his transition into strategic leadership roles that bridge science and business. With a proven track record in the CRO and CDMO sectors, Angel now serves as Director of Business Development at endPoints, leading growth through strategic partnerships and global market expansion.
Why LATAM
High Recruitment and Retention Rates
▾- Treatment-naïve populations
- Strong doctor-patient relationships increase trial retention.
- Fast enrollment speeds accelerate timelines.
Diverse and Representative Populations
▾- Genetically and ethnically diverse populations.
- Ideal for trials requiring global applicability or inclusion of underrepresented groups.
- High endemicity of tropical and vector-borne diseases.
- Outbreak patterns and climate allow for seasonal targeting and real-world efficacy trials.
- High prevalence of infectious and chronic diseases.
Cost-Effectiveness
▾- Lower operational and labor costs compared to the US/EU.
- Favorable USD exchange rates make LATAM financially attractive.
Strong Medical Infrastructure and Regulatory Evolution
▾- Modern hospitals and trained investigators, especially in urban centers.
- Regulatory agencies operate in an optimized manner and are aligned with global standards.
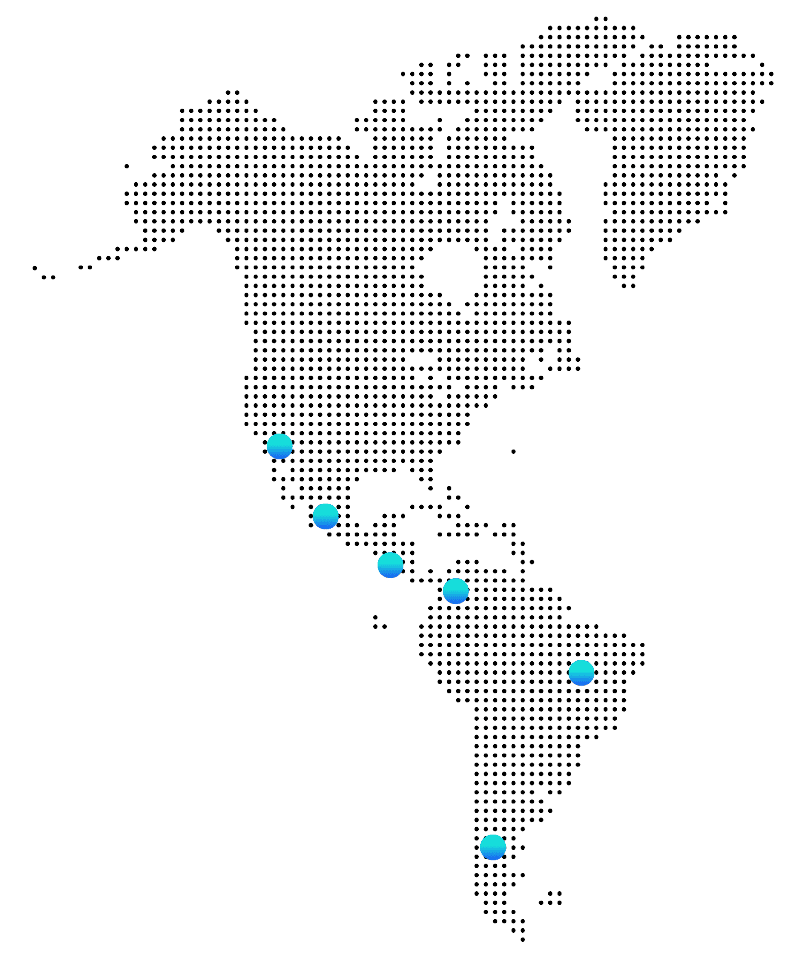
Why LATAM
High Recruitment and Retention Rates
▾- Treatment-naïve populations
- Strong doctor-patient relationships increase trial retention.
- Fast enrollment speeds accelerate timelines.
Diverse and Representative Populations
▾- Genetically and ethnically diverse populations.
- Ideal for trials requiring global applicability or inclusion of underrepresented groups.
- High endemicity of tropical and vector-borne diseases.
- Outbreak patterns and climate allow for seasonal targeting and real-world efficacy trials.
- High prevalence of infectious and chronic diseases.
Cost-Effectiveness
▾- Lower operational and labor costs compared to the US/EU.
- Favorable USD exchange rates make LATAM financially attractive.
Strong Medical Infrastructure and Regulatory Evolution
▾- Modern hospitals and trained investigators, especially in urban centers.
- Regulatory agencies operate in an optimized manner and are aligned with global standards.

Our Technology
Smart Tech. Smart Trials
Leveraging advanced technology to streamline processes, optimize data, and enchance clinical outcomes
Data transparency is real-time within the organization and for the sponsors and CROs.
Increased collaboration and trust in the organization thanks to technology-enabled processes and data in a single system—no need to implement a data warehouse or APIs between different vendors.
Examples of Customized Dashboards
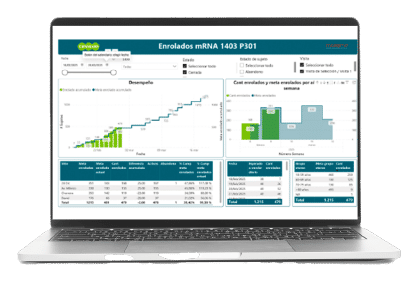
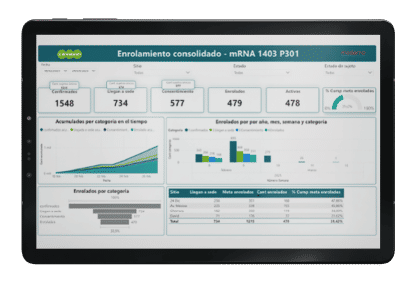
Our Technology
Smart Tech. Smart Trials
Leveraging advanced technology to streamline processes, optimize data, and enchance clinical outcomes
Data transparency is real-time within the organization and for the sponsors and CROs.
Increased collaboration and trust in the organization thanks to technology-enabled processes and data in a single system—no need to implement a data warehouse or APIs between different vendors.
Examples of Customized Dashboards


